Original Article
Antibacterial
and Anticandidal Activity of Ethanolic Extracts of Pomegranate (Punica granatum
Linn) Peel, Sudan
Saga T. Ahmed1, Abdelgadir A. Abdelgadir2,
Bakri Y. M. Nour3, Asma H. Abuaisha4, Elhadi A. Ahmed1*
Introduction: Pomegranate fruit is grown in Sudan
and used in folk medicine to treat various diseases. This study aimed to assess
the antimicrobial activity of the ethanol extract against common clinically
important bacteria and Candida albicans.
Methods: Pomegranate (Punica granatum Linn) peel was purchased from the local market in Wad Medani, Gezira State. The extraction was carried out by alcoholic maceration and examined against standard strains of Staphylococcus aureus and Klebsiella pneumonae, and clinical isolates of Escherichia coli and Candida albicans. The well-diffusion method was used for antimicrobial testing.
Results: Different concentrations of extracts; 200, 100, 50, 25, 12.5, 6, 3 and 1.5 mg/ml, exhibited strong antibacterial and anticandidal activities. The maximum diameter of inhibition zones was observed against gram negative bacteria; Escherichia coli followed by Klebsiella pneumonae.
Conclusion: The current finding could highlight the Pomegranate fruit as a potential source of new antimicrobial agents.
Keywords: Pomegranate peel, Antibacterial, Anticandidal, Ethanolic extract.
________________________________________________________________________
1Department of Medical Microbiology, Faculty of Medical Laboratory Sciences, University of Gezira, Sudan.
2Department of Pharmacognosy, Faculty of Pharmacy, University of Gezira, Wad Medani, Sudan.
3Department of Medical Parasitology, Faculty of Medical Laboratory Sciences, University of Gezira, Sudan
4Department of Community Medicine, Faculty of Medicine, Almughtaribeen University, Khartoum, Sudan
*Correspondent author: Elhadi A. Ahmed, Department of Medical Microbiology, Faculty of Medical Laboratory Sciences, University of Gezira, Sudan. Email: hadilabone@yahoo.com, hadilabone@uofg.edu.sd
To download a full PDF copy click here ⏬
Introduction:
Bacterial infections greatly impact human life, especially with the growing resistance of many of these microorganisms to antibiotics. Studies have shown that children’s deaths due to infections are mainly caused by methicillin-resistant Staphylococcus aureus and extended-spectrum beta-lactamase producers of gram-negative bacilli.(1) In recent decades, fungi have become another important cause of diseases, and Candida albicans was considered the most common fungal pathogen.(2) Treatment of fungal diseases is generally difficult due to the presence of a degree of similarity between fungi and human cells and for this reason, there are small numbers of antifungals available.(3) In addition, drug resistance by C. albicans has become a matter of concern.(4,5,6) In the current scenario of the emergence of multiple drug resistance of human pathogens, it is crucial to discover new antimicrobial agents with diverse chemical constituents, novel mechanisms of action against problematic infectious diseases and fewer side effects. Failure in treatment and increased drug resistance encouraged scientists to search for alternative medicinal plants, guided by community practices.(7) Many plants synthesize compounds that have vital and beneficial functions to humans and animals and may have defensive properties against microorganisms.(8)
Punica granatum Linn belongs to the Punicaceae family and known as Pomegranate, has recently been described as the force fruit of nature and was used in folk medicine to treat many illnesses.(9) The specific components of this plant are multiple, including flavonoids, polyphenols, tannins and organic acids. It also has antioxidant, anti-inflammatory, anticancer and antiviral activities.(10) Methanol is the most common solvent used to extract pomegranate, other solutions used to a lesser degree include water, ethanol and acetone.(11) This study aimed to assess the antimicrobial activity of ethanol extract of Sudanese pomegranate peel against common clinically significant gram-positive and gram-negative bacteria and C. albicans.
Materials and Methods:
Study design
Experimental laboratory-based study conducted at the Faculty of Medical Laboratory Sciences and Faculty of Pharmacy, University of Gezira, Wad Medani, Sudan.
Microorganisms studied
Laboratory strains of Staphylococcus aureus ATCC (25923) and Klebsiella pneumonae ATCC (13833); as well as clinical isolates of Escherichia coli and Candida albicans identified by Medical Laboratory, University of Gezira were used. Strains were sub-cultured and purified on Nutrient, MacConkey and Sabouraud's dextrose agars.
Plant preparation and extraction
Pomegranate fruit peels were purchased from the Wad Medani City’s local market, Gezira State. The peels were washed thoroughly with distilled water and then shade-dried for 4 days. The dried plant material (79.11 g) was powdered using an electric blender into a coarse powder. The powder was soaked in 500 ml of ethanol (70%) for 7 days with occasional shaking. The extract was then filtered and evaporated using rotary evaporator. The extract was stored in in a refrigerator in an air tight, dark glass bottle (Figure 1). Different concentrations of extract were prepared for testing as follow: 200 mg/ml, 100 mg/ml, 50 mg/ml, 25 mg/ml, 12.5 mg/ml, 6 mg/ml, 3 mg/ml and 1.5 mg/ml.
Figure 1: Process and extraction of pomegranate peel
1: Pomegranate peel, 2: Dried materials, 3: Dissolved powder, 4: Extracts.
Preparation of McFarland standard turbidity (0.5) for microbial inoculums suspension
Barium sulfate was used to adjust the turbidity of the inoculums. It was prepared by adding a 1% v/v sulfuric acid solution to 1.17% w/v barium chloride solution. The turbidity standard was prepared by adding 0.6 ml of barium chloride to 99.4ml of the sulfuric acid solution.(12)
Antimicrobial sensitivity testing (Well diffusion method)
Bacterial colonies were suspended in sterile normal saline (0.9%) and modified to yield suspension containing about 1010 Colony Forming Units (CFU) (compared with 0.5 McFarland standard turbidity). About 25 ml of nutrient agar medium was poured into the plates and 200 μl of bacterial suspension was added to the agar plates. Plates were gently mixed to achieve equal distribution of bacteria in agar. After the complete solidification of the agar, four circular wells, 8 millimeters in diameter, were cut using a sterile cork borer. Two wells were filled with 200μl of the Ethanolic Extracts of Pomegranate Peel (EEPP/EPPE) while the other wells were filled with 100 μl of 50 μg/ml ceftriaxone as positive control and 100 μl of distilled water as negative control. The plates were left at room temperature for 40 min and then incubated in the upright position overnight at 35-37°C.(13, 14)
The Fungal strain was grown in Sabouraud's dextrose agar for 48 hours, then harvested and washed off with sterile normal saline to produce a suspension match to 0.5 McFarland standards containing approximately 2.5 x 106 CFU/ml.
Anti-fungal standard 25 μg/ml Nystatin was used as a positive control.
Interpretation of results
Results were stated in diameters of inhibition zones in millimeters using the following standard: < 9 mm zone was considered inactive; 9-12 mm partially active while 13-18 mm was active and > 18 mm was very active. (15, 16)
Results:
Using an agar well-diffusion test, examined extracts showed varying levels of antimicrobial activities against the targeted microorganisms.
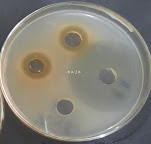
Figure2. Well-diffusion test: left, K. pneumoniae at 200 mg/ml EPPE, Right, Escherichia coli at 6mg/ml EPPE.
Figure 2 shows examples of the effectiveness of ethanolic extract on tested microorganisms with different concentrations. Note the large size of Inhibition Zone (IZ) when the highest concentration was used (200 mg/ml) against K. pneumoniae. On the other hand, note the small size of IZ when tested with a concentration of 6 mg/ml against another organism which is E. coli.
E. coli showed the highest susceptibility with a mean zone of inhibition of 34 mm at a 200 mg/ml concentration and 13 mm at a concentration of 1.5 mg/ml as shown in Table (1). This means that the EPPE is effective down to the lowest concentration used.
Table 1. Means of Inhibition Zones of Ethanolic Extract of Pomegranate Peel against E. coli
References:
(1) Miller-Petrie, Molly, et al. “Drug-Resistant Infections.” Major Infectious Diseases, edited by King K Holmes et. al., 3rd ed. The International Bank for Reconstruction and Development / The World Bank. 2017.
(2) Hani U, Shivakumar HG, Vaghela R, Osmani RA, Shrivastava A. Candidiasis: a fungal infection–current challenges and progress in prevention and treatment. Infect Disord Drug Targets. 2015;15(1):42–52.
(3) Endo, E.H., Cortez, D. A., Ueda-Nakamura, T., Nakamura, C.V., Dias Filho, B. P. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Research in microbiology. 2010; 161(7), 534–540.
(4) Prasad, R., Nair, R., Banerjee, A. Emerging Mechanisms of Drug Resistance in Candida albicans. Progress in molecular and subcellular biology. 2019; 58, 135–153.
(5) Toda, M., Williams, S. R., Berkow, E. L., Farley, M. M., Harrison, L. H., Bonner, L., Marceaux, K. M., Hollick, R., Zhang, A. Y., Schaffner, W., Lockhart, S. R., Jackson, B. R., & Vallabhaneni, S. Population-Based Active Surveillance for Culture-Confirmed Candidemia - Four Sites, United States, 2012-2016. Morbidity and mortality weekly report. Surveillance summaries. 2019; 68(8), 1–15.
(6) Costa-de-Oliveira, S., Rodrigues, A. G. Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms. 2020; 8(2), 154.
(7) Sangeetha J, Vijayalakshmi K. Antimicrobial activity of rind extracts of Punica granatum Linn. The Bioscan. 2011;6:119-24.
(8) Wardasha Belal. Comparison Between the Antifungal Activities of Extracts of four Different plants (Pomegranate, Garad, Moleata and Morenga). Mster thesis. 2016; URI:http://repo.uofg.edu.sd/handle/123456789/1479.
(9) Lim T. K. (2012). Punica granatum. Edible Medicinal And Non-Medicinal Plants: Volume 5, Fruits. 2012; (5) 136–194.
(10) Madugula, P., Reddy, S., Koneru, J., Rao, A. S., Sruthi, R., & Dalli, D. T. "Rhetoric to Reality"- Efficacy of Punica Granatum Peel Extract on Oral Candidiasis: An in vitro Study. Journal of clinical and diagnostic research: JCDR. 2017; 11(1), ZC114–ZC117.
(11) Dahham, S.S.; Ali, M.N.; Tabassum, H.; Khan, M. “Studies on antibacterial and antifungal activity of pomegranate (Punica granatum L.),” American-Eurasian Journal of Agricultural & Environmental Sciences. 2010; 9(3):273–281.
(12) Cheesbroug, M. District Laboratory Practice in Tropical Countries infection Part 2. 2ed edition. Cambridge University Press, New York, USA. 2006; 137,179.
(13) Magaldi, S., Mata-Essayag, S., Hartung de Capriles, C., Perez, C., Colella, M. T., Olaizola, C., Ontiveros, Y. Well diffusion for antifungal susceptibility testing. International journal of infectious diseases: IJID : official publication of the International Society for Infectious Diseases. 2004; 8(1), 39–45.
(14) Daoud, A., Malika, D., Bakari, S., Hfaiedh, N., Mnafgui, K., Kadri, A., Gharsallaha, N. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of date palm pollen (DPP) from two tunisian cultivars. Arabian Journal of Chemistr.2019; 12 (8): 3075-3086.
(15) Valgas, C.; De Souza, S.M.; Smânia, E.F.A.; Smânia Jr, A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007; 38:369-380.
(16) Mukhtar, S. and Ifra Ghori. Antibacterial activity of aqueous and ethanolic extracts of garlic, cinnamon and turmeric against Escherichia coli ATCC 25922 and Bacillus subtilis DSM 3256.” International Journal of Applied Biology and P. 2012; (3): 131-136.
(17) Nduche M.U; Iwuoha C.D; Igbokwe A.U. Antibacterial activity of four Nigerian medicinal plants. Scholars Journal of Agriculture and Veterinary Sciences. 2016; 3(3):172-180.
(18) Oraki, H. H.; Demirci, A. Ş.; Gümüş, T. Antibacterial and antifungal activity of pomegranate (Punica granatum L.CV.) Peel. Electronic Journal of Environmental, Agricultural & Food Chemistry. 2011; 10(3):1958-1969.
(19) Al-Zoreky N. S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. International journal of food microbiology. 2009; 134(3), 244–248.
Behera, S., Khetrapal, P., Punia, S. K., Agrawal, D., Khandelwal, M., & Lohar, J. Evaluation of Antibacterial Activity of Three Selected Fruit Juices on Clinical Endodontic Bacterial Strains. Journal of pharmacy & bioallied sciences. 2017; 9(Suppl 1), S217–S221.
(20) Moorthy, K., Punitha, T., Vinodhini, R., Sureshkumar, B. T., Vijayalakshmi, P., Thajuddin, N. Antimicrobial activity and qualitative phytochemical analysis of Punica granatum Linn. (PERICARP). Journal of Medicinal Plants Research. 2013; 7(9):474-479.
(21) Jaisinghani, R. N., Makhwana, S., Kanojia, A. Study on antibacterial and flavonoids content of ethanolic extract of Punica granatum (pomegranate) peel. Microbiology research. 2018; 9:7480.
(22) Yehia, H. M., Elkhadragy, MF., Moneim, A. E. A. Antimicrobial activity of pomegranate rind peel extracts. African Journal of Microbiology Research. 2011; 4(22):3664-366.
(23) Gullón, P., Astray, G., Gullón, B., Tomasevic, I., Lorenzo, J. M. Pomegranate Peel as Suitable Source of High-Added Value Bioactives: Tailored Functionalized Meat Products. Molecules (Basel, Switzerland). 2020; 25(12), 2859.
(24) Fawole, O. A., Makunga, N. P., Opara, U. L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC complementary and alternative medicine.2012; 12, 200.
(25) Chen, J.; Liao, C.; Ouyang, X.; Kahramanoğlu, I.; Gan, Y.; Li, M. Antimicrobial Activity of Pomegranate Peel and Its Applications on Food Preservation, Journal of Food Quality, 2020; Article ID 8850339, 8 pages.

%20(1).jpg)